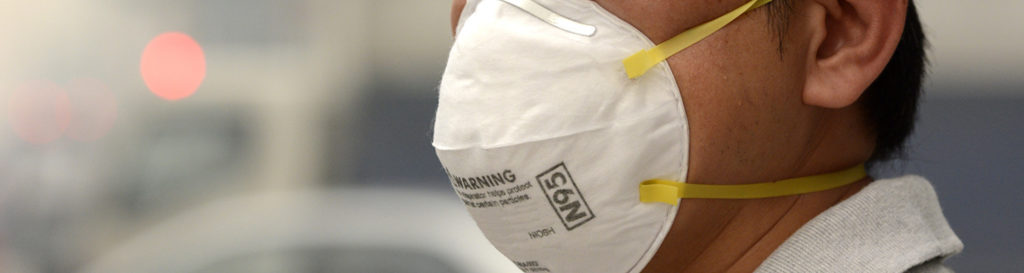
HIGH FILTERABILITY | EXCELLENT PROTECTION
A universal, one-size respirator, both with and without exhalation valve are designed to fit most face shapes and sizes while providing outstanding respiratory protection. Reliable, value-priced protection against a wide range of non-oily particles. Engineered to provide 95% NIOSH filtration efficiency.
- Designed to be an economical choice for effective respiratory protection.
- 95% filtration efficiency against solid particulates and non-petroleum based liquid aerosols.
- Recommended for protection against many substances including pandemic diseases such as H5N1 Avian Flu and SAR
- This Particulate Respirator N95 is an American standard managed by NIOSH – part of the Center for Disease Control (CDC).
- This Particulate Respirator N95 is FDA approved and CE approved.
PRODUCT FEATURES
- Respirators are tight fitting masks, designed to create a facial seal
- Non-valve respirators provide good two-way protection, by filtering both inflow and outflow of air
- These are designed protect the wearer (when worn properly), up to the safety rating of the mask
TECHNICAL SPECIFICATIONS
| ITEM | TEST METHOD | STANDARD | TEST RESULT | INCLUDE |
| Surface Moisture Resistance | GB/T 4745-1997 | ≥ 3 | 1 # 4 2 # 4 3 # 4 | Qualified |
| Synthetic Blood Penetration Resistance | GB 19083-2010 5.5 Pressure:10.7kPa | No penetration | No penetration | Qualified |
| BFE(%) | GB 19083-2010.5.4 Gas flow:85L/min Aerosol particle: NaCl Aerosol load: 15mg/n3? Temperature: 23.1℃ Relative humidity: 36.8% | ≥ 95 | Min 99.852 | Qualified |
| Airflow Resistance (Pa) | GB 19083-2010.5.4 Gas flow:85L/min Aerosol particle: NaCl Aerosol load: 15mg/n3? Temperature: 23.1℃ Relative humidity: 36.8% | ≤343.2 | Max 224.4 | Qualified |
PARTICULATE RESPIRATOR-KN95/ FFP2

HIGH FILTERABILITY I EXCELLENT PROTECTION
Filtering facepiece respirators (FFR) provide a much higher level of protection with face form fitting and greater pressure drop. It is designed to fit most face shapes and sizes while providing outstanding respiratory protection. Reliable, value-priced protection against a wide range of non-oily particles. Engineered to provide 95% filtration efficiency.
- Designed to be an economical choice for effective respiratory protection.
- 95% filtration efficiency against solid particulates and non-petroleum based liquid aerosols.
- Recommended for protection against many substances including pandemic diseases such as H5N1 Avian Flu and SAR
- This Particulate Respirator KN95/FFP2 is FDA approved and CE approved.
- The Center for Disease Control (CDC) recommends the KN95/FFP2 for use in high risk situations, especially by medical personnel during pandemics.
PRODUCT FEATURES
- Respirators are tight fitting masks, designed to create a facial seal
- Non-valve respirators provide good two-way protection, by filtering both inflow and outflow of air
- These are designed protect the wearer (when worn properly), up to the safety rating of the mask.
- Excellent for filtering non-oil-based particles such as those resulting from wildfires, PM 2.5 air pollution, volcanic eruptions, or bioaerosols (e.g. viruses).
TECHNICAL SPECIFICATIONS
| ITEM | CONDITIONS | STANDARD |
| Test Standard | GB 2626-2006 | |
| Basic Information | Respiratory protection equipment, Self-inhalation air-purifying particulate respirator KN type is for filtering non-oil particle KP type is for filtering both non-oil particle and oil particle | |
| Application | Self-inhalation filter type respiratory, which is applicable to the protection of all kinds of particulate matter | |
| BFE | Gas flow:85L/min | Level 1:≥ 95% Level 2:≥ 99% Level 3:≥ 99.97% |
| PFE | Particle size: median diameter of aerosol particle size distribution:0.075μm±0.020μm, Gas flow:85L/min | Non-oil particle(N) KN 90 ≥ 90% KN 95 ≥ 95% KN 100 ≥ 99.97% Oil Particle(P) KP 90 ≥ 90% KP 95 ≥ 95% KP 100 ≥ 99.97% |
| Total Inspiratory Resistance | ≤ 350 Pa | |
| Total Expiratory Resistance | ≤ 250 Pa |
SURGICAL MASK- 3PLY